Conjugated compounds
Conjugated compounds
Aromatic compounds
•Benzene causes a depressed white blood cell count on prolonged exposure and should not be used as a laboratory solvent
•Fractional distillation of coal tar yields benzene, toluene, xylene, naphthalene, and a host of other aromatic compounds such as Figure 1
•Unlike coal, petroleum contains few aromatic compounds and consists largely of alkanes. During petroleum refining, however, aromatic molecules are formed when alkanes are passed over a catalyst at about 500℃ under high pressure.
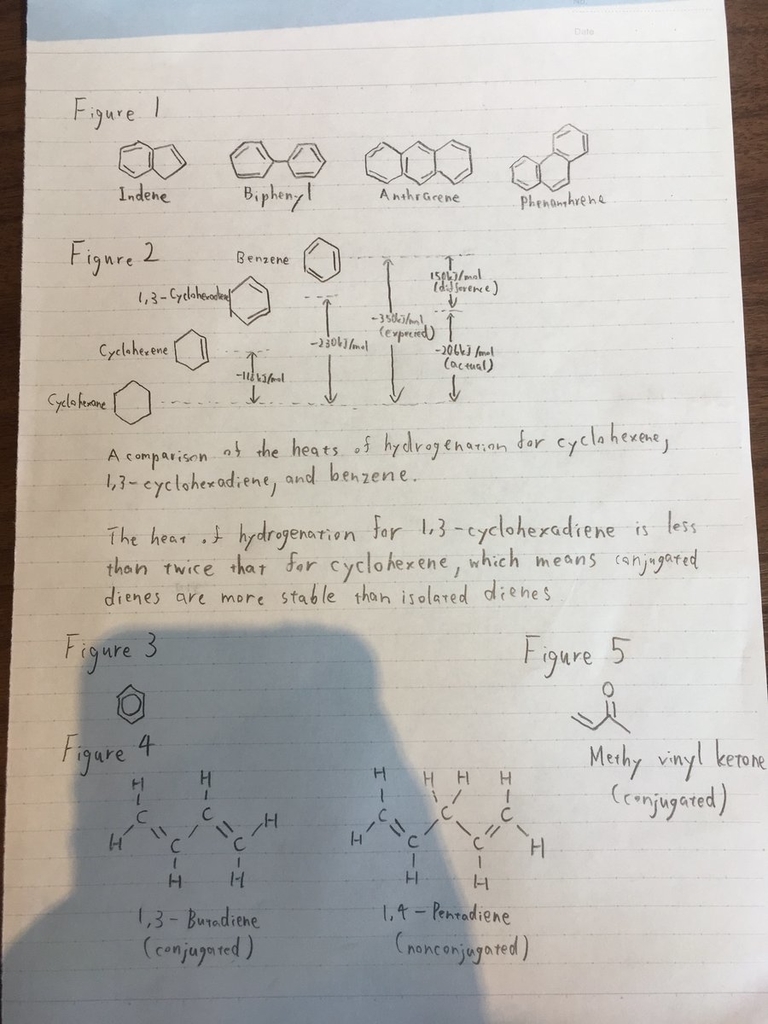
•benzene’s stability can be explained by measuring heats of hydrogenation(see Figure 2 for more information)
•benzene is sometimes represented like figure 3. however, it doesn’t indicate the number of π electrons in the ring.
1,3-Butadiene(see Figure 4)
•this is the example of compounds that have different unsaturations close together which interact with one another.
•1,4-Pentadiene is nonconjugated(see Figure 4)
enones
•an enone is a type of organic compound consisting of an alkene conjugated to a ketone.
•progesterone, the hormone that prepares the uterus for implantation of a fertilized ovum is also a conjugated enone
•the simplest enone is methyl vinyl ketone(see figure 5)
conjugated polyene
•lycopene, the red pigment found in tomatoes and thought to protect against prostate cancer, is a conjugated polyene
How does the cell prevent mutation?
How does the cell prevent mutation?
Unintended chemical changes such as the formation of thymine dimer, depurination, and deanimation affect DNA replication in a bad way, causing the substitution of one nucleotide pair for another and the deletion of nucleotide pairs in the daughter DNA strand. Cells need to prevent those mutations from occurring, but how?
There are two cases, depending on which the cell determines what kind of measure to take. The first case is when only nucleotides on one strand are damaged. In this case, they can be repaired using the information present in the complementary strand in two different ways. The first one is to cleave the covalent bonds that join the damaged nucleotides to the rest of the DNA strand by nucleases or other enzymes(the type of enzyme used in this reaction changes depending on the situation), then fill in the gap by making a complementary copy of the information stored in the undamaged strand using repair DNA polymerases, and lastly seal the nick by DNA ligase. Given that most DNA damage creates structures that are never encountered in an undamaged DNA strand, it is easy for these enzymes involved in these steps to differentiate between the good strands and the bad. The second way is called mismatch repair. The system restores the correct sequence, removing a portion of the DNA strand containing the error and resynthesizing the missing DNA. This way is effective only in the case of newly synthesized DNA.
(For your information)
An inherited predisposition to certain cancers is caused by mutations in genes that encode mismatch repair proteins. Humans inherit two copies of these genes from each parent. If one inherits two damaged mismatch repair genes, they cannot prevent mutations by mismatch repair and thus have a higher possibility to get cancer. Humans who inherit one damaged mismatch repair gene is unaffected until another mismatch repair gene is mutated in a somatic cell.
The second case is that both strands are damaged. Cells adopt two ways against this case: nonhomologous end joining and homologous recombination. Nonhomologous end joining is carried out by nucleases which clean the broken ends and by DNA ligase which rejoins them. However, this mechanism have a weakness in that nucleotides are often lost in the phase of broken end cleaning. Homologous recombination mostly occurs shortly after a cell’s DNA has been replicated before cell division. A nuclease digests 5’ ends of broken strands, and then with the help of specialized enzymes, one of the broken 3’ ends invades the unbroken homologous DNA duplex, and DNA ligation follows after a repair DNA polymerase resynthesizing DNA.
The cell duplicates its DNA at rates as high as 1000 nucleotides per second, but how? There are several kinds of protein that help DNA replication.
The cell duplicates its DNA at rates as high as 1000 nucleotides per second, but how? There are several kinds of protein that help DNA replication.
The DNA helicase sits at the very front of the replication machine(on the lagging strand template) where it uses the energy of ATP hydrolysis to propel itself forward, prying apart the double helix as it speeds along the DNA. Because each hydrogen bond is individually weak, it can unzip the double helix at normal temperatures. Moreover, DNA has more AT base pairs at replication origins, for they form less hydrogen bonds than CG base pairs, and thus are weaker.
DNA polymerase catalyzes the addition of nucleotides to the 3’ end of a growing DNA strand. The energy needed in this reaction is provided by the hydrolysis of the incoming nucleotide itself. Then, pyrophospate, which arises in the hydrolysis, is further hydrolyzed to inorganic phosphate to make the polymerization reaction effectively irreversible. On top of that, DNA polymerase has another two special qualities to minimize the number of replication errors. The first one is only when the match(AT or CG) is correct, does it catalyze the nucleotide addition reaction(because GT and CA can also be formed). The second one is the proofreading system with a nuclease. When DNA polymerase makes a rare mistake and adds the wrong nucleotide, it clips off the mispaired nucleotide and tries again. Polymerization and proofreading are carried out by different catalytic domains in the same polymerase molecule. Hence, 5’ to 3’ direction is for proofreading. Also, DNA polymerases don’t proofread RNA primers because RNA stands out as suspect copy to be automatically removed and replaced by DNA.
On the lagging strand template, single strand DNA binding proteins cling to the single stranded DNA exposed by the helicase, transiently preventing the strands from re-forming base pairs and keeping them in an elongated form so that they can serve as efficient templates.
DNA topoisomerases produce transient nicks in the DNA backbone, which temporarily release the tension. They can reseal the nick before falling off the DNA.
A sliding clamp keeps DNA polymerase firmly attached to the template while it is synthesizing new strands of DNA. The clamp loader hydrolyzes ATP each time it locks a sliding clamp around a newly formed DNA double helix.
For your information,
•On the lagging strand template, RNA primer will be erased by nucleases, a repair polymerase replaces it with DNA and then DNA ligase works and links the two Okazaki fragments.
•The slower rate of fork movement in eukaryotes(including humans) may be due to the difficulties in replicating DNA through the more complex chromatin structure of eukaryotic chromosomes
my next biology essay is probably about the end replication problem
Many different activated carriers that help cell metabolism
Many different activated carriers that help cell metabolism
ATP
In my last essay, I mentioned how enzyme catalyzed reactions, which is energetically unfavourable, occur in the cell. However, there is another way to promote energetically unfavourable reactions in the cell: ATP. ATP is one of the most famous activated carriers and promotes condensation reactions using its energy stored in the phosphoanhydride bond(because when ATP hydrolysis occurs ΔG is negative). This process occurs when it generates other activated carriers, or links subunits together by bonds formed during the condensation reaction(and produces the nucleic acids, proteins and polysaccharides).
NADPH and NADH
They carry energy in the form of hydride ions(two high energy electrons plus a proton). The difference between them is that they differ in conformation, which makes it possible to bind as substrates to different sets of enzymes. Thus, they have different rolls. NADH is used in catabolic reactions which occurs when the cell is synthesizing ATP. On the other hand, NADPH is needed in anabolic reactions. In other words, NADH acts as a oxidizing agent while NADPH acts as a reducing agent. Basically, inside the cell the ratio of NAD+to NADH is kept high whereas the ratio of NADP+to NADPH is kept low(for the reason above)
Acetyl CoA
This is also one of the activated carriers but i won’t explain it cuz i don’t know much about it!(but it was kind of surprising when i found out it is an activated carrier) it carries acetyl groups.
In activated carriers, the transferable group makes up only a small part of the molecule. the rest serves as a convenient handle facilitating the recognition of the carrier molecule by specific enzymes. This handle mostly contains a nucleotide, which may be a relic from an early stage of cell evolution(it is said the main catalysts for early life forms on earth were RNA molecules)
The relation of thermodynamics with the metabolism of the cell
The relation of thermodynamics with the metabolism of the cell(I suck at explaining things)
There are two sets of reactions that constitute the metabolism of the cell: anabolism and catabolism. Those reactions are promoted by enzymes. To what kind of reaction do they pertain? And how?
According to the second law of thermodynamics, in the universe or in any isolated system, the degree of disorder can only increase. We can express this statement in terms of probability by saying that systems will change spontaneously toward those arrangements that have the greatest probability. For example, consider a box of 100 coins all lying heads up. If you keep jiggling the box, the ratio of heads to tails changes toward 50 to 50 because of the probability.
We can explain how enzyme related biochemical reactions are performed by the second law of thermodynamics. Basically, while energetically favourable reactions spontaneously proceed until they reach a state of equilibrium(because the degree of disorder increases), enzyme related reactions are energetically unfavourable, and thus cannot take place by itself. Then, how do they occur? We can measure the amount of disorder by the free energy change, denoted ΔG. Free energy is needed to increase disorder. Thus, when ΔG is negative, it is easy for energetically favourable reactions to occur, and when ΔG is positive, energetically unfavourable reactions are promoted. Enzyme related reactions take place when they are coupled to a second reaction with a negative ΔG large enough that the net ΔG of the entire process is negative.